by Bill Snyder
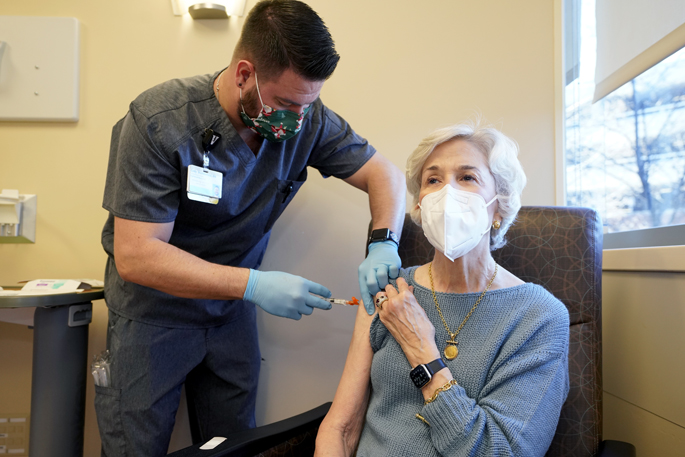
On Dec. 22, Caroline Davis of Nashville became the first patient at Vanderbilt University Medical Center to receive injections of a new antibody combination to protect her from COVID-19.
The antibodies were discovered by James Crowe, MD, Robert Carnahan, PhD, and their colleagues in the Vanderbilt Vaccine Center. Six antibodies were licensed in June 2020 to AstraZeneca for optimization and advancement into clinical trials.
Davis, who is being treated for cancer at VUMC, said she could not produce antibodies against the COVID-19 virus on her own, despite receiving two doses of a COVID-19 vaccine and a booster, because the chemotherapy she is receiving suppresses her immune system.
“It’s very exciting,” she said, before Cody Stubblefield, RN, gave her two injections of the antibodies, one in each arm, in an outpatient clinic at VUMC. “This is the best Christmas present that could possibly have been given to me.”
The long-acting antibody combination, called Evusheld, was discovered at VUMC and developed by the global biopharmaceutical company AstraZeneca. On Dec. 8, the U.S. Food and Drug Administration approved Evusheld for emergency use to prevent COVID-19 in adults and children 12 years and older.
The therapy is authorized only for people who are not currently infected with the COVID-19 virus and who are immunocompromised because of medical conditions or treatment for disorders including cancer or who have a history of a severe adverse reaction to a COVID-19 vaccine.
Last week the U.S. Department of Health and Human Services began distributing the first allotment of doses to state health departments around the country. VUMC was among the first to receive doses from Tennessee’s allotment of about 1,000 doses, officials said.
Davis’ physician, David Morgan, MD, said, “In patients for whom the vaccine doesn’t work, this is a great option.” Morgan, associate professor of Medicine in the Division of Hematology and Oncology, estimated that hundreds of patients being treated for cancer at VUMC could benefit from Evusheld.
The injections provide about six months of protection from COVID-19 with only minor side effects such as rash, headache and mild fever. Preclinical data released this week by AstraZeneca suggest that Evusheld is protective against the omicron variant of COVID-19, which is spreading rapidly around the world.
Evusheld currently is the only antibody combination given by intramuscular injection to protect uninfected people from COVID-19. Other antibody combinations are given by intravenous infusion to patients who have recently been infected to prevent them from becoming seriously ill.
Because of the limited supply, Evusheld will be given only to the highest risk patients, including transplant recipients and those being treated for cancer. Patients who qualify should contact their physicians, who will order the injections and place them on a list to receive them as soon as possible.
Evusheld is not an option for people who are not immunocompromised or who have not had a severe adverse reaction to a COVID-19 vaccine. For most people, “vaccination is still the primary prevention,” Morgan said.
One of the studies showed that a long-acting, two-antibody combination reduced the risk of COVID-19 symptoms at six months by 83% compared to inactive placebo.
The VUMC research was supported by the Defense Advanced Research Projects Agency of the U.S. Department of Defense, the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, and the Dolly Parton COVID-19 Research Fund at Vanderbilt.